Tetraphase Pharmaceuticals Inc. biopharmaceutical products. The Company offers antibiotics to treat multi-drug resistant bacterial infections. Tetraphase Pharmaceuticals serves patients in the United States.
Tetraphase Pharmaceuticals Inc. filed patent application numbered 2502/DELNP/2012 that is titled as TETRACYCLINE COMPOUNDS. This patent application has been granted as Patent Number 316574.
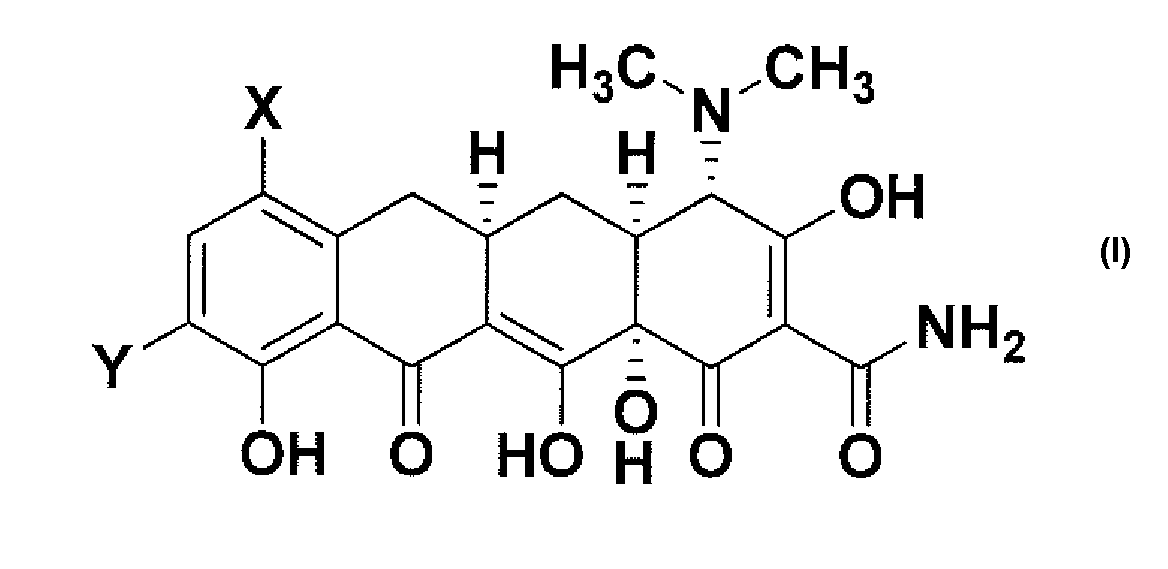
The invention covers Chemical compound. The invention is directed to a compound represented by Structural Formula (1): or a pharmaceutically acceptable salt thereof. The variables for Structural Formula (I) are defined herein. Also described is a pharmaceutical composition comprising the compound of Structural Formula (I) and its therapeutic use.
During patent examination, the patent examiner raised objections under Section 3(d) of the Indian Patents Act that Claims 1-8 fall under section 3(d) of the Patents (Amended) Act, 2005 as the said claims defines new use and/or new form of the known compound (as cited by the prior art documents as described in the report). In the absence of experimental data, it is not clear if the claimed compound and the composition thereof act to provide an enhancement of the known efficacy i.e., demonstrate a greater technical effect and/or differ significantly in properties w.r.t the known compound.
As a response to the said objection, the applicant submits that only those compounds which are new form of a known substance will fall within the purview of Section 3(d) and for that it is necessary to the following should be present:
a) a known substance,
b) a new form of that substance and;
c) the new form in order to be patentable has to demonstrate therapeutic efficacy, which has to be established by means of filing comparative data. The words “other derivatives” ought to be interpreted ejusdem generis and every new compound cannot be treated as a derivative of any earlier compound. It is only new forms of compounds which are derived from the same known compound or substance i.e., a new form of a known entity (Section 2(ta)) that would attract the rigors of Section 3(d). The new novel tetracycline compounds do not fall in that explanation. Further structural difference is mentioned.
Advocate Rahul Dev is a Patent Attorney & International Business Lawyer practicing Technology, Intellectual Property & Corporate Laws. He is reachable at rd (at) patentbusinesslawyer (dot) com & @rdpatentlawyer on Twitter.
Quoted in and contributed to 50+ national & international publications (Bloomberg, FirstPost, SwissInfo, Outlook Money, Yahoo News, Times of India, Economic Times, Business Standard, Quartz, Global Legal Post, International Bar Association, LawAsia, BioSpectrum Asia, Digital News Asia, e27, Leaders Speak, Entrepreneur India, VCCircle, AutoTech).
Regularly invited to speak at international & national platforms (conferences, TV channels, seminars, corporate trainings, government workshops) on technology, patents, business strategy, legal developments, leadership & management.