Bial Portela & Cª S.A. is an innovative pharmaceutical establishment, devoted to determining, manufacturing, and commercializing medicines, to enhance people’s lives throughout the world. Founded in 1924, BIAL formulates, develops, and provides novel therapeutic solutions.
In India, the Chemical business of Bial Portela & Cª S.A. focuses on processes for preparing medicaments for the treatment of cardiovascular diseases and intermediates for use therein, a process for preparing 1,3-dihydroimidazole-2-thione derivatives, urea compounds and their use as enzyme inhibitors and process.
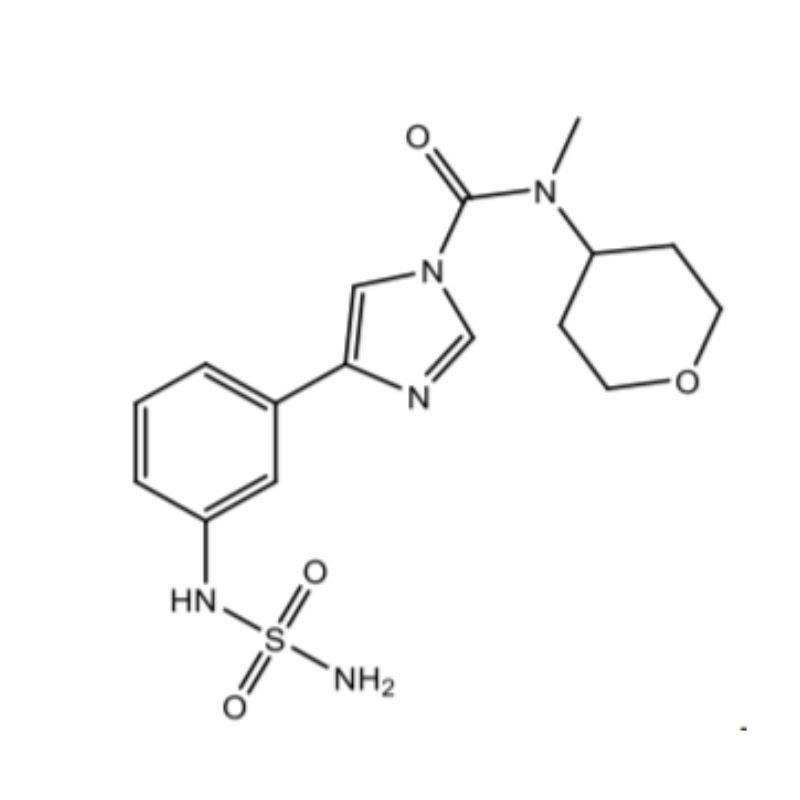
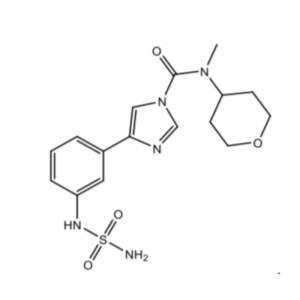
Bial Portela & Cª S.A. filed a patent application numbered 201617002593 that is titled as IMIDAZOLECARBOXAMIDES AND THEIR USE AS FAAH INHIBITORS. The patent has been filed in the field of Chemicals. This Patent Application has been granted as Patent Number 346464. This invention covers a compound having a structure selected from a pharmaceutically acceptable salt thereof. The compound can be utilized as an inhibitor of fatty acid amide hydrolase.
During the patent examination, the patent examiner raised objections under Sections 3(d), 3(e), and 3(i) of the Indian Patents Act, 1970. Under Section 3(d) the examiner objected that the claims relate to a mere derivation of known compounds and lacks efficacy data of said compounds over the prior arts. Under section 3(e) the objection stated that the claimed composition appears to have been obtained by a mere admixture resulting only in an aggregation of the properties of the components without any synergistic effect. Finally, under Section 3(i) the objection stated that the dosage form is a method of treatment of human beings.
As a response, the Applicant made individual submissions for each objection. Under Section 3(d) submitted that without identifying the specific ‘known substance’, neither can the Controller assert that the claimed compounds are new forms of such ‘known’ substance, nor can the Applicant accurately respond to the same. Next, it was submitted that the pending claims are directed towards a composition comprising a novel compound. Compositions comprising at least one novel component do not fall within the scope of Section 3(e) of the Act. Lastly, under Section 3(i) the Applicant respectfully disagreed with the objection raised by the examiner and submitted that the abstract filed at the time of applying is already in a prescribed manner. Therefore, the examiner was requested to take the above on record and withdraw the objections.
Advocate Rahul Dev is a Patent Attorney & International Business Lawyer practicing Technology, Intellectual Property & Corporate Laws. He is reachable at rd (at) patentbusinesslawyer (dot) com & @rdpatentlawyer on Twitter.
Quoted in and contributed to 50+ national & international publications (Bloomberg, FirstPost, SwissInfo, Outlook Money, Yahoo News, Times of India, Economic Times, Business Standard, Quartz, Global Legal Post, International Bar Association, LawAsia, BioSpectrum Asia, Digital News Asia, e27, Leaders Speak, Entrepreneur India, VCCircle, AutoTech).
Regularly invited to speak at international & national platforms (conferences, TV channels, seminars, corporate trainings, government workshops) on technology, patents, business strategy, legal developments, leadership & management.