BioCryst Pharmaceuticals, Inc., incorporated on November 15, 1991, is a biotechnology company. The Company designs, optimizes and develops small molecule drugs that block enzymes involved in the pathogenesis of diseases. The Company focuses on the treatment of rare diseases. The Company uses X-ray crystallography, computer modeling of molecular structures and chemistry techniques to focus on the three-dimensional molecular structure and active site characteristics of the enzymes that control cellular biology. The Company’s drug candidates include RAPIVAB, RAPIACTA, ALPIVAB, PERAMIFLU, Avoralstat, BCX7353, other second generation hereditary angioedema (HAE) compounds, Galidesivir and Forodesine.
BioCryst Pharmaceuticals, Inc. filed patent application numbered 8012/DELNP/2011 that is titled USEFUL PHARMACEUTICAL SALTS OF 7-[(3R 4R)-3-HYDROXY-4-HYDROXYMETHYL-PYRROLIDIN-1-YLMETHYL]-3 5-DIHYDRO-PYRROLO[3 2-D]PYRIMIDIN-4-ONE. This patent application has been granted as Patent Number 312836.
The invention covers the field of pharmaceuticals. The invention provides novel hemi- and mono-salts of 7-[(3R 4R)-3-Hydroxy-4- hydroxymethyl-pyrrolidin-1-ylmethyl]-3 5-dihydro-pyrrolo[3 2-d]pyrimidin-4-one (Compound 1) with various organic and inorganic acids. In one embodiment the organic acid is a C4 organic diacids. The present disclosure further provides novel methods for preparing these salts. The novel monohydrates hemisalts of the C4 organic diacids are isostructural and can be prepared with different properties. Multiple acids can be used simultaneously and the proportion of acids can be varied offering the opportunity to select hemi-salts of compound 1 with desired properties.
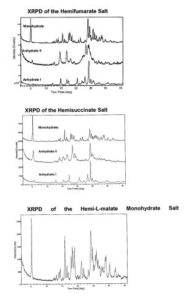
During patent examination, the patent examiner raised objections under Section 3(d) of the Indian Patents Act, wherein the examiner stated that the Claim(s) (1-4) are statutorily non-patentable and mere discovery of a new form of a known substance which does not result in the enhancement of the known efficacy of that substance or the mere discovery of any new property or new use for a known substance or of the mere use of a known process. Derivatives of known substance shall be considered to be the same substance, unless they differ significantly in properties with regard to efficacy.
As a response, the applicant presented technical arguments along with revised claims to the patent examiner.
Advocate Rahul Dev is a Patent Attorney & International Business Lawyer practicing Technology, Intellectual Property & Corporate Laws. He is reachable at rd (at) patentbusinesslawyer (dot) com & @rdpatentlawyer on Twitter.
Quoted in and contributed to 50+ national & international publications (Bloomberg, FirstPost, SwissInfo, Outlook Money, Yahoo News, Times of India, Economic Times, Business Standard, Quartz, Global Legal Post, International Bar Association, LawAsia, BioSpectrum Asia, Digital News Asia, e27, Leaders Speak, Entrepreneur India, VCCircle, AutoTech).
Regularly invited to speak at international & national platforms (conferences, TV channels, seminars, corporate trainings, government workshops) on technology, patents, business strategy, legal developments, leadership & management.

